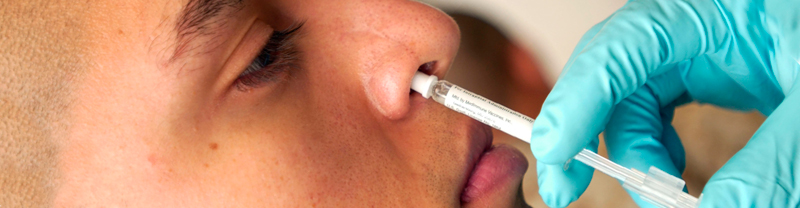
Many parents and children love the FluMist flu vaccine because it avoids the necessity of receiving another shot. FluMist is approved by the Food and Drug Administration for people between the ages of 2 and 49 as an effective and safe way to guard against the flu virus. A flu shot contains an inactivated influenza vaccine; however, FluMist contains live viruses that have been weakened. FluMist stimulates the immune system so that it can fight against the flu virus if it encounters that strain in the future.
In a surprise turn, the CDC has now recommended against using FluMist. After approving FluMist in 2003, the CDC preferred FluMist for children under the age of 8 because it appeared to perform better than a flu shot. However, poor performance in recent years is a concern for the CDC and the FDA. FluMist is only about 46% effective while the flu shot remains about 65% effective.
A drawback of FluMist according to one pediatrician is a runny nose for a couple of days. Other common side effects of FluMist are similar to side effects of a flu shot including low fever, sore throat, cough, muscle pain, headache, fatigue, irritability, and vomiting. At this time it is a recommendation not a drug recall; therefore, the recommendation could be changed for next year’s flu season should the drug manufacturer is able to show better performance.
All Drugs Have Side Effects and Pose Risks to Patients
Whether it is an over-the-counter medication or a prescription drug, all drugs have side effects and pose certain risks for patients. Not all patients will experience side effects; however, the risk is still the same. Most side effects are discovered during the testing and trial phase of a new drug. Subjects are given the drug and side effects are monitored.
According to the FDA, some side effects may be predicted based on what occurred during the testing and trial phase but “rare events occur unexpectedly when many more people take the drug after it is approved.” Patients can find information about potential side effects and risks by visiting the FDA’s website.
One way to help the FDA and doctor’s understand the side effects and warn others of the potential risks is for patients and doctors to report side effects to the FDA. You can file a report online, by fax, mail, or telephone.
What if I am Injured by a Defective Drug?
If a drug manufacturer fails to follow proper procedures with developing, testing, and manufacturing a drug, the company can be held liable if the drug injures a patient. Furthermore, if a manufacturer or distributor fails to provide adequate instructions for dosage or fails to provide information regarding potential risks and side effects, the company can be held liable for any injures to patients.
If you believe you have been injured by a defective drug, you need to contact the defective drug attorneys of The Olinde Firm as soon as possible. You may be entitled to receive compensation for your injuries but you must act quickly. You only have a limited time to file a claim.
Contact our office at (504) 587-1440 or 1-800-587-1889 for a free case evaluation.