IVC Filter Complications
Home »
New Orleans BARD IVC Filter Attorneys
IVC (Inferior vena cava) filter
What is An IVC Filter?
An Inferior Vena Cava (IVC) Filter is a small, medical device that looks like a tiny cage. The device is inserted into a patient’s veins to act as a filter that blocks blood clots from reaching the lungs. Several serious side effects and complications have been reported by patients suffering vein or organ perforations, heart damage, lung damage, and internal bleeding as a result of the IVC Filter breaking apart and migrating throughout the body.
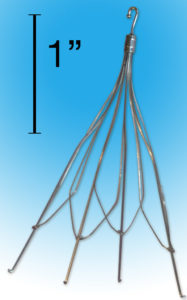
When or Why Is an IVC Filter Used?
Most patients at risk of developing blood clots are administered blood thinners by their physicians. For patients who are unable to take blood thinners, a retrievable IVC Filter is typically used to keep blood clots from entering the lungs. The IVC Filters are surgically implanted into a patient’s veins and are meant to be retrievable.
How Does the IVC Filter Work?
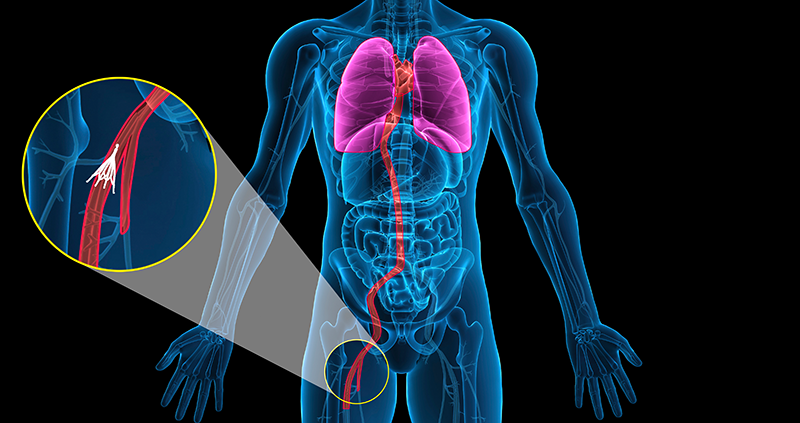
The largest vein in the body is called the inferior vena cava. This vein is responsible for delivering de-oxygenated blood to the heart and lungs. The retrievable IVC Filter is surgically implanted by way of a catheter into the inferior vena cava through an incision in the neck or groin area. The IVC Filter catches any formed blood clots before they enter the right atrium of the heart, or later, the lungs.When the filter is no longer needed, it is retrievable. Again, through an incision, a device is inserted into the patient which catches a small hook at the top of the device, then the filter is covered by a protective sheath and removed through the incision.
FDA Notes Complications Associated With The Use of Retrievable IVC Filter Device
By 2005, the FDA had received 921 reports of complications which included:
- Migration of the IVC Filter
- Perforation of the Vein or Internal Organs
- Breaking of the Device or Fracturing of Components
- Device Components Detaching
- Embolization
328 (35.6%) of the complications involved the device detaching and migrating. The second most common complication listed by the FDA was embolization, whereby pieces of the device break off and become lodged in the body, causing damage to internal organs, internal bleeding, and possibly death.
The FDA announced a safety alert in 2010 regarding retrievable IVC Filters. In 2013, those warnings were confirmed in the findings of a research study by the Journal of American Medical Association (JAMA). That study indicated that a mere 58 devices out of 679 (8%) were removed successfully. They also found that most IVC Filters were left in patients longer than necessary.
Did You Suffer Complications From an IVC Filter?
If you suffered any harmful side effects or complications from a BARD retrievable IVC Filter, we strongly urge you to contact our Defective Medical Device attorneys right away. We are currently offering a Free Consultation to help you make an informed decision. There are time limits to file claims against the manufacturer of the IVC Filters, get started today with your Free Consultation.
FREE CASE REVIEW
If you or a loved one has been injured by the negligence of others, please contact our law firm today. You have rights and may be entitled to compensation for your injuries.
New Orleans
Of Counsel at Huber, Thomas & Marcelle
1100 Poydras Street, Suite 2200
New Orleans, LA 70163
Phone: (504) 587-1440
Toll Free:1-800-587-1889
North Shore
Mailing Address
P.O. Box 55
Mandeville, LA 70470
Phone: (504) 587-1440
Toll Free: 1-800-587-1889
New Orleans East
New Orleans East Office
8816 Chef Menteur Highway
Suite B
New Orleans, LA 70127
Phone: (504) 587-1440
Toll Free: 1-800-587-1889
Free Case Evaluation
Copyright © 2025 The Olinde Firm - New Orleans Attorneys. All rights reserved. Disclaimer The information contained in this website may be considered advertising under the Rules of the Supreme Judicial Court of Louisiana and is for general information purposes only. Nothing contained within this or associated pages, documents, emails, or other communications should be taken as legal advice for any individual case or situation. The information on this website is not intended to create, and communications exchanged in connection with this website are not intended to create or constitute an attorney-client relationship. Although all communications are considered confidential, we do not accept representation of clients until such time as we enter into a written agreement for such representation.
