Essure Birth Control Complications
Home »
Essure Birth Control Complications
New Orleans Attorney for Essure Side Effects
Essure® Birth Control Contraceptive Linked To Serious Side Effects, Complications, Revisionary Surgery, Pregnancies and Deaths
The Essure® birth control device is a non surgical permanent contraceptive that is popular with women who want to avoid possible complications inherent with invasive surgery. The Essure® device consists of two flexible metal coils which are placed in the fallopian tubes. The coils contain polyester fibers that actually cause inflammation in the fallopian tubes. This inflammation is supposed to stop sperm flow into the tubes, and prevent contraception.
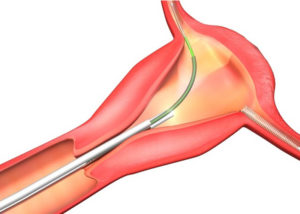
Ironically, many women have had to undergo revisionary surgery or a total hysterectomy as a result of serious side effects and complications linked to the Essure® device.
Complications Linked To The Essure® Contraceptive Device
- Allergic Reactions (to the nickel used in the coil)
- Migration of the coil through the fallopian tubes
- Fallopian tube perforation
- Uterus perforation
- Hysterectomy
- Vaginal Bleeding
- Cramping
- Nausea
- Fainting
- Vomiting
- Unusual or Ectopic pregnancies
- Back pain
- Pelvic pain
Complications from the Essure birth control device can lead to very serious injury and possibly death. The Food & Drug Administration (FDA) has been asked to ban the Essure™ birth control device by several members of Congress, stating the benefits of the device do not outweigh the inherent risks involved.
If you suffered serious complications as a result of an Essure® birth control device, call us toll free today! 1-800-587-1889
PA Congressman Introduces E-Free Act to Ban Essure & Revoke FDA Approval
Essure Contraceptive Device The Target Of Legislation For FDA Ban
Congressman Mike Fitzpatrick (R-PA) introduced a bill urging a ban the on the Essure device from the market. Citing over 22,000 women who have come forward (on Facebook alone) reporting complications and harmful side effects after receiving the Essure Sterilization device. A grassroots organization has also been formed by women experiencing problems from the Essure® device.
Over 5,000 Formal Complaints Registered With FDA
The FDA has received more than 5,000 complaints from women who were harmed or injured by the Essure© permanent birth control device. Several deaths, including four adults and five fetus’, have been linked to complications of an Essure© device. The fetus deaths all involved pregnancies that the Essure© device was supposed to prevent.
Did You Suffer Complications From An Essure® Birth Control Device?
If you received an Essure® contraceptive device and suffered serious complications, you may be entitled to compensation. Make an informed decision about your legal rights by scheduling a free consultation with a medical device attorney at the Olinde Firm today.
FREE CASE REVIEW
If you suffered any serious complications from an Essure® birth control device, please contact us now for a free, confidential consultation.
New Orleans
Of Counsel at Huber, Thomas & Marcelle
1100 Poydras Street, Suite 2200
New Orleans, LA 70163
Phone: (504) 587-1440
Toll Free:1-800-587-1889
North Shore
Mailing Address
P.O. Box 55
Mandeville, LA 70470
Phone: (504) 587-1440
Toll Free: 1-800-587-1889
New Orleans East
New Orleans East Office
8816 Chef Menteur Highway
Suite B
New Orleans, LA 70127
Phone: (504) 587-1440
Toll Free: 1-800-587-1889
Free Case Evaluation
Copyright © 2020 The Olinde Firm - New Orleans Attorneys. All rights reserved. Disclaimer The information contained in this website may be considered advertising under the Rules of the Supreme Judicial Court of Louisiana and is for general information purposes only. Nothing contained within this or associated pages, documents, emails, or other communications should be taken as legal advice for any individual case or situation. The information on this website is not intended to create, and communications exchanged in connection with this website are not intended to create or constitute an attorney-client relationship. Although all communications are considered confidential, we do not accept representation of clients until such time as we enter into a written agreement for such representation.
